Step-by-step explanation:
The given reaction equation is as follows.
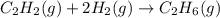
Let us assume that the partial pressure of
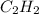 be 'x' atm. Hence, the partial pressure of
be 'x' atm. Hence, the partial pressure of
 = (0.1 - x) atm
= (0.1 - x) atm
And, amount of
 left unreacted is as follows.
left unreacted is as follows.
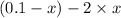 = 0.1 - 3x
= 0.1 - 3x
Now, the pressure of
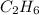 = partial pressure of reacted
= partial pressure of reacted
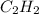 = x
= x
So, the pressure of product will be as follows.
Pressure of the product = partial pressure of
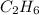 + partial pressure of unreacted
+ partial pressure of unreacted

0.042 = x + (0.1 - 3x)
2x = 0.058
or, x = 0.029 atm
Therefore, mole fraction of
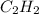 = partial pressure of
= partial pressure of
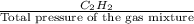
=
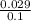
= 0.29
Thus, we can conclude that the mole fraction of acetylene in the original mixture is 0.29.