Answer:
Molarity = 0.0907 moles/liter
What is Molarity?
Molarity is the measure of how many moles of a solute are dissolved in a quantity of a solution. The quantity of solution is typically expressed in liters. It can also be seen as the concentration of moles in a solution.
The formula for molarity is
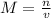 or
or
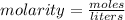 . To solve a problem like this one, you'll need to use dimensional analysis to get all of your numbers into the correct units.
. To solve a problem like this one, you'll need to use dimensional analysis to get all of your numbers into the correct units.
Calculating the Number of Moles
Let's start off by figuring out the number of moles of aluminum nitrate present. The first thing we'll need is the molecular weight of the compound.
The chemical formula for aluminum nitrate is Al(NO₃)₃. So, we need to add up the weights of one Al, three Ns, and nine Os. You can find the weights, given in grams per mole, on the periodic table.
26.982 + (3 x 14.007) + (9 x 15.999) = 212.994 g/mol
Using the molecular weight of the compound and the given amount of grams in the problem, we can determine how many moles of the compound are in the solution.
Set this up using the two fractions shown below. If it helps you keep track of which unit you're going to end up with, you can cross off units that cancel out on the tops and bottoms of the fractions. For example, since "grams" is in the top of one and the bottom of the other, it cancels out, and you're left with only "moles" to be the answer's unit.
To solve, multiply everything across the top, then everything across the bottom, and divide them by each other.
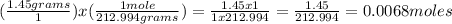
There are 0.0068 moles of aluminum nitrate in the solution.
Calculating the Amount of Solvent
In the problem, we're told we have 75 mL of solution. To convert this into liters, we can use the same method from above.
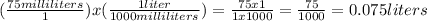
We have 0.075 liters of solution. Now, we can plug both of these numbers into the molarity formula.
Calculating the Molarity
Molarity =
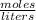
Molarity =
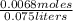
Molarity = 0.0907 moles/liter (rounded up in the ten-thousandths place)
So, for every one liter we have of this solution, there will be 0.0907 moles of aluminum nitrate dissolved in it.