Answer:
The order of increasing bond lenght :
-C≡C < -C=C < -C-C
Step-by-step explanation:
Bond length is defined as average distance between two nuclei of bonded atoms in a molecule.Bond length is inversely proportional to the number of bonds present between two atoms.
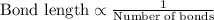 ...[1]
...[1]
Bond energy is defied as amount of energy required to break apart the bond of 1 mole of molecule into their individual atom.
Bond energy is directly proportional to the number of bonds present between two atoms.
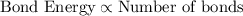 ..[2]
..[2]
From [1] and [2]:
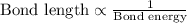
Bond : Energy (kJ/mol)
-C-C : 346
-C=C : 612
-C≡C : 835
The order of decreasing bond energy :
835 kJ/mol > 612 kJ/mol > 346 kJ/mol
The order of increasing bond lenght :
-C≡C < -C=C < -C-C