Answer:
1020g
Step-by-step explanation:
Volume of can=
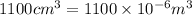
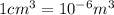
Mass of can=80g=
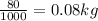
1Kg=1000g
Density of lead=
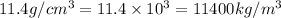
By using
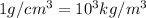
We have to find the mass of lead which shot can it carry without sinking in water.
Before sinking the can and lead inside it they are floating in the water.
Buoyancy force =
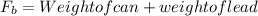
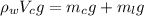
Where
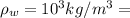 Density of water
Density of water
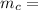 Mass of can
Mass of can
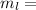 Mass of lead
Mass of lead
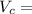 Volume of can
Volume of can
Substitute the values then we get
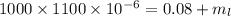
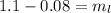
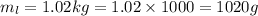
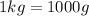
Hence, 1020 grams of lead shot can it carry without sinking water.