Answer:
Mass= 314.96 grams
Step-by-step explanation:
The density of the substance is defined as mass per unit volume of it.
The formula is :
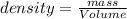
Unit conversion:
1 liter = 1000 ml
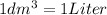
So
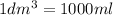 ....(1)
....(1)
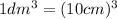
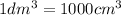
Replace The dm3 by cm3 in (1)
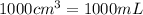
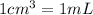
Volume = 254 mL = 254 cm3
Calculation of mass
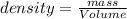
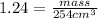
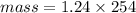
Mass= 314.96 grams(or 315 grams)
Note: Always makes same units before solving the question. You can not determine the mass if Volume is in mL and Density is in g/cm3. Change mL into cm3 first