Step-by-step explanation:
In order to calculate the number of moles of a compound, we will find it out as follows.
No. of moles of chromium(III) chloride =
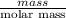
=
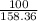
= 0.63 moles
No. of moles of
 =
=
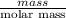
=
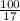
= 5.88 moles
No. of moles of
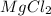 =
=
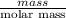
=
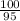
= 1.05 moles
No. of moles of
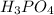 =
=
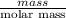
=
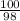
= 1.02 moles
No. of moles of KCl =
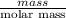
=
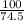
= 1.34 moles
No. of moles of
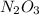 =
=
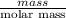
=
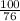
= 1.31 moles
No. of moles of
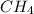 =
=
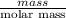
=
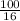
= 6.25 moles
No. of moles of
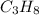 =
=
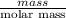
=
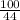
= 2.27 moles
No. of moles of
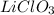 =
=
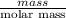
=
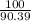
= 1.11 moles
Therefore, we can conclude that out of the given options
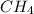 contains the largest number of moles of compound.
contains the largest number of moles of compound.