Answer:
The empirical formula of this question is :
C.
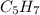
Step-by-step explanation:
The empirical formula is the simplest whole number ratio of elements in the chemical formula of the molecule.
It is calculated using:
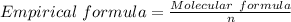
n = The common factor
The Molecular formula represents the exact composition of the elements in the compound.
For example :
Molecular formula : C4H8O2
Find the common factor n ?
here , 2 is common factor(which is divisible by 4 ,8,2)
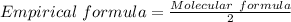
divide each element by 2
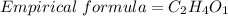
generally 1 is not written
C2H4O is the empirical formula
In this question , the molecular formula C40H56
n = common factor = 8(It is divisible by 40 , 56 )
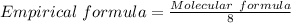
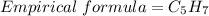
So, correct option is C