Answer: The equilibrium constant for the given reaction is
![K_a=([BrO^-][H_3O^+])/([HBrO])](https://img.qammunity.org/2021/formulas/chemistry/college/a5l2fkfgzuiug600be9shpoyzfrphdymx8.png)
Step-by-step explanation:
Equilibrium constant is defined as the ratio of concentration of products to the concentration of reactants each raised to the power their stoichiometric ratios. It is expressed as
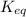
For a general chemical reaction:
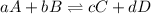
The expression for
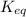 is written as:
is written as:
![K_(eq)=([C]^c[D]^d)/([A]^a[B]^b)](https://img.qammunity.org/2021/formulas/chemistry/college/jbd7uq80565lgtnpqjte86knmd4rxyi07m.png)
The concentration of pure liquids and pure solids are taken as 1.
The chemical equation for the reaction of hypobromous acid with water follows:
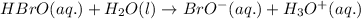
The expression of equilibrium constant for above equation follows:
![K_a=([BrO^-][H_3O^+])/([HBrO])](https://img.qammunity.org/2021/formulas/chemistry/college/a5l2fkfgzuiug600be9shpoyzfrphdymx8.png)
Hence, the equilibrium constant for the given reaction is
![K_a=([BrO^-][H_3O^+])/([HBrO])](https://img.qammunity.org/2021/formulas/chemistry/college/a5l2fkfgzuiug600be9shpoyzfrphdymx8.png)