Answer:
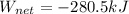
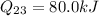
Step-by-step explanation:
The net work of the cycle is the sum of works in each process.
For the first process 1-2: Let's apply the work definition.
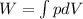 (1)
(1)
Now, we need the pressure. We know that pV=C, where C is a constant. Then
![p_(1)V_(1)=10^(5)2=2*10^(5) [J]](https://img.qammunity.org/2021/formulas/physics/college/gbfgamvkswdsi8xdngrya1zepqeox9izee.png)
So
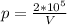
Let's put p in (1):
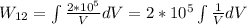
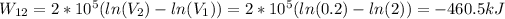
Using the first law of thermodynamics we can find Q.
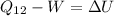
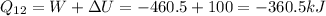
Second process 2-3
In this case, we have a constant volume, so the work done here is 0.
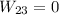
Third process 3-1
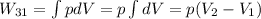
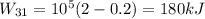
Finally, the net work is:
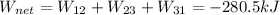
By the conservation of energy:
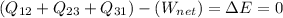
Because there is no change in total energy.
So:
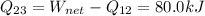
It is a refrigerator because the net work is negative, it means it consumes energy.
I hope it helps you!