Answer:
a) k = 0.0417 M⁻¹.s⁻¹
b) Ea = 49.4 kJ/mol
Step-by-step explanation:
a) If the decomposition reaction is a second-order reaction, the rate law is:
rate = k*[NOCl]²
The rate is how much the concentration decays per second (M/s), thus, if its initially 0.300 M and it decays 15%, the final concentrations 85% of 0.300, which is 0.255 M. The time needed for that is 0.20 min = 12 s, so:
rate = (0.300 - 0.255)/12
rate = 0.00375 M/s
Thus, the rate constant is:
0.00375 = k*(0.300)²
0.09k = 0.00375
k = 0.0417 M⁻¹.s⁻¹
b) By the Arrhenius equation, the constant k is related to the activation energy as:
k =
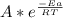
Where A is a constant, R is the gas constant (8.31 J/mol.K) and T is the temperature. If we apply ln in the equation:
ln(k) = ln(A) - Ea/RT
Thus, for T1 = 15°C (288 K), k = k1, and for T = 25°C (298 K), k = k2, knowing that Ea and A are independent of the temperature:
ln(k1) = ln(A) - Ea/RT1
ln(k2) = ln(A) - Ea/RT2
ln(k1) - ln(k2) = ln(A) - ln(A) - Ea/RT1 + Ea/RT2
ln(k1/k2) = (Ea/R) * (1/T2 - 1/T1)
If the rate constant doubles, k2 = 2k1
ln (1/2) = (Ea/8.31) * (1/298 - 1/288)
-0.6932 = (Ea/8.31)*(-1.1652x10⁻⁴)
1.402x10⁻⁵Ea = 0.6932
Ea = 49438.84 J/mol
Ea = 49.4 kJ/mol