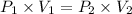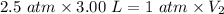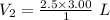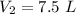Answer:
7.5 L
Step-by-step explanation:
At constant temperature and number of moles, Using Boyle's law
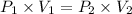
Given ,
V₁ = 3.00 L
V₂ = ?
P₁ = 36.74 psi = 2.5 atm (Conversion factor, 1 psi = 0.068046 atm)
P₂ = 1 atm (Atmospheric pressure as it comes to surface)
Using above equation as: