Answer: The mass percentage of carbon element is 63.57 %, hydrogen element is 5.96 %, nitrogen element is 9.28 % and oxygen element is 21.19 %
Step-by-step explanation:
We are given:
A chemical compound having chemical formula of
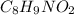 . This compound is made by the combination of carbon, hydrogen, nitrogen and oxygen elements.
. This compound is made by the combination of carbon, hydrogen, nitrogen and oxygen elements.
1 mole of acetaminophen contains 8 moles of carbon atom, 9 moles of hydrogen atom, 1 mole of nitrogen atom and 2 moles of oxygen atom
Mass of carbon element =
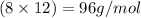
Mass of hydrogen element =
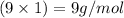
Mass of nitrogen element =
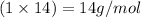
Mass of oxygen element =
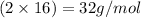
Mass of the compound = 151 g/mol
To calculate the percentage composition of an element in a compound, we use the equation:
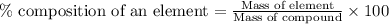 ........(1)
........(1)
Putting values in equation 1, we get:
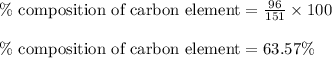
Putting values in equation 1, we get:
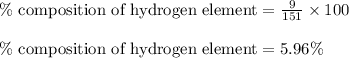
Putting values in equation 1, we get:
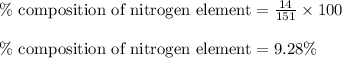
Putting values in equation 1, we get:
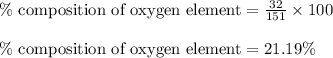
Hence, the mass percentage of carbon element is 63.57 %, hydrogen element is 5.96 %, nitrogen element is 9.28 % and oxygen element is 21.19 %