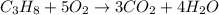Answer:
The correct answer is option 4.
Step-by-step explanation:
Hydrocarbons are defined as molecule composed of carbon and hydrogen atoms.They are of two types:
Saturated hydrocarbons : These are the hydrocarbons in which only single bond between carbon- carbon atoms is present.
Unsaturated hydrocarbons : These are the hydrocarbons in which double or triple bond between carbon- carbon atoms is present.
Combustion reaction is defined as the chemical reaction in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide gas and water molecule.
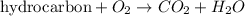
For example: