To solve this problem we will apply the first law of thermodynamics and we will make a balance between the heat transferred, its internal energy and the total work. Recall that for gases the definition of work can be expressed in terms of its pressure and volume. Let's start
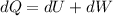
Here,
dU = Internal Energy
dW = Work
But internal energy is unchanged, then
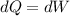
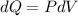
Where
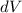 = Change in Volume
= Change in Volume
P = Pressure
Finally, the expression of the heat transferred can be expressed in terms of pressure and volume, so it would end up becoming
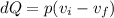
Replacing,
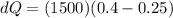
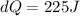
Therefore the correct answer is B.