To solve this problem we will use the concepts given in Coulomb's laws where the electrostatic force of a body is defined. In this case on an electron in a Uranium atom. Recall that in a neutral state the uranium atom has 92 electrons. The magnitude of the force on an electron is
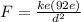
Here,
k = Coulomb's Constant
e = Charge of electron
d = Distance between them
Replacing we have that,
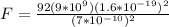
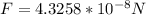
Therefore the force on this electron is

Now the magnitude of the force on an electron is
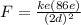
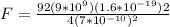
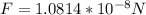
If the distance of the elctron from the nucleus were double the force would be
