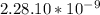To solve this problem it is necessary to apply the concepts related to the force constant of HF with energy excitation. Said expression may be mathematically encompassed as follows.
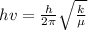
Here
h = Planck's Constant
v = Frequency of radiation required for excitation
k = Force constant
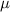 = Reduced mass of the molecule
= Reduced mass of the molecule
The relative population of two energy levels is expressed by the relation as follows
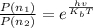
Here
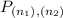 = Population of energy at each level
= Population of energy at each level
 = Boltzmann constant
= Boltzmann constant
T= Temperature of the molecule (300K at our case)
Calculate the reduced mass of HF molecule as follows,
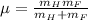
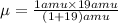
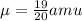
Convert the reduced mass in terms of kg,
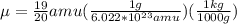
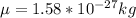
Now the excitation energy for two energy states would be
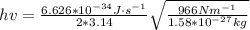
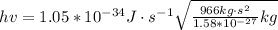
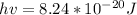
Then calculating the relative population of two energy states we have that
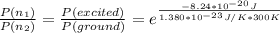
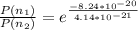
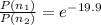
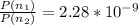
Therefore the relative population of excited stated to ground energy state is