Answer:
The emoirical formula for the given compound is :
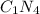 . It is simply written as CN4
. It is simply written as CN4
Step-by-step explanation:
Molecular formula : It is the symbolic representation of chemical composition of different elements present in the molecule.
For example : Benzene is C6H6
It is the true composition of the elements.
Empirical Formula :It is the simple whole number ratio of the elements present in the given molecule.
For example :
The empirical formula of Benzene is:
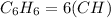
= CH
Steps to reach at the empirical formula :
- Search the common factor if any in the formula .
- divide the whole formula by the common factor.
In C3N12
3 is the common factor
divide each term by 3
C3 on dividing by 3 = C1
N12 on dividing by 3 = N4
Hence the empirical formula of the compound is CN4