Answer:
As any molecular or ionic compound, 0 net charge, as a hypothetical diatomic cation, 2+
Step-by-step explanation:
The question states that this is a compound, and any molecular or ionic compound would have a net charge of 0.
However, such a compound doesn't exist. Francium generally exists in nature having a 1+ charge in its cation form. Combining two francium ions would result in a
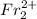 species which is simply a diatomic ion but not a compound.
species which is simply a diatomic ion but not a compound.