Answer:
The molar concentration of
 in the solution is
in the solution is
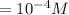 and Predicted molar concentration of
and Predicted molar concentration of
 in the solution is
in the solution is
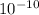 M
M
Step-by-step explanation:
Given :
![pH = - log[H^(+)]\\](https://img.qammunity.org/2021/formulas/biology/middle-school/jbhayh70swzgvl2ukrktfjheweis5mjglq.png)
= 4
Molar concentration of
 in the solution is:
in the solution is:
![[H^(+)]=10^(-pH)\\](https://img.qammunity.org/2021/formulas/biology/middle-school/tu5o9654w3lp8zfu6w8v5z5yrrfda4ny6i.png)
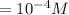
![[H^(+)][OH^(-)] = k_(w)](https://img.qammunity.org/2021/formulas/biology/middle-school/8gjxb1dbfvmqbguyvchf1azwqppo50mcoa.png)
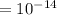
Predicted molar concentration of
 in the solution is:
in the solution is:
![[OH^(-)] =([10^(-14)])/([H^(+)])](https://img.qammunity.org/2021/formulas/biology/middle-school/jbjyt5tu6h67oah45ceoqwruogyrue7wnl.png)
=
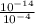
=
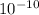 M
M