Answer : Yes, the following nuclear reaction are balanced.
Explanation :
Beta emission or beta minus decay : It is a type of decay process, in which a neutrons gets converted to proton, an electron and anti-neutrino. In this the atomic mass number remains same.
The beta minus decay equation is represented as,
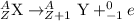
(A is the atomic mass number and Z is the atomic number)
The given reaction follow beta minus decay as:
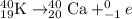
Hence, the following nuclear reaction are balanced.