Answer:
0.68
Step-by-step explanation:
Both
 and
and
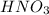 are strong acids which ionize completely in water. According to stoichiometry, 1 mole of each will produce 1 mole of hydronium cations. Therefore, let's calculate the total number of moles of hydronium and find the final concentration of it keeping in mind the dilution:
are strong acids which ionize completely in water. According to stoichiometry, 1 mole of each will produce 1 mole of hydronium cations. Therefore, let's calculate the total number of moles of hydronium and find the final concentration of it keeping in mind the dilution:
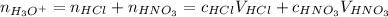
Total molarity:
![[H_3O^+] = (c_(HCl)V_(HCl) + c_(HNO_3)V_(HNO_3))/(V_(HCl) + V_(HNO_3))](https://img.qammunity.org/2021/formulas/chemistry/middle-school/9dqq9d796aicanb6xjy1n7kj0n1mc4lmxt.png)
Find pH:
![pH = -\log[H_3O^+] = -\log((c_(HCl)V_(HCl) + c_(HNO_3)V_(HNO_3))/(V_(HCl) + V_(HNO_3))) = -\log((0.30~M\cdot 20.00~mL + 0.15~M\cdot 30.00~mL)/(20.00~mL + 30.00~mL)) = 0.68](https://img.qammunity.org/2021/formulas/chemistry/middle-school/3pesvw5x1137b29r49eow1p1qsap5zfw8q.png)