The density of the product gas mixture is 5.39 g/L
Step-by-step explanation:
Given:
Temperature after the reaction =
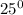 C
C
Pressure in the container = 1.65 atm
The ideal gas equation PV = nRT
To find: Density of the product gas mixture
Step 1:
Molarity (M) = P/(RT)
![\[M=(P)/(R T)\]$M=\frac{1.65 \mathrm{atm}}{\left(0.08206 (L \cdot a t m)/(m o l . K)\right)(298.15 K)}$](https://img.qammunity.org/2021/formulas/chemistry/middle-school/6j79l8iimkppsh6p31wx04wmhlovh49bnh.png)
M = 0.0674 mol/L
Step 2:
The product of the given reaction is
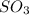 . Its molar mass is
. Its molar mass is
1S x 32.066 g/mol = 32.066 g/mol
3O x 16 g/mol = 48 g/mol
Adding both we get 80.066 g/mol
Therefore the density of the product gas mixture is
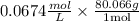
= 5.39 g/L