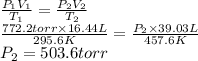Answer:
b) 503.6 torr
Step-by-step explanation:
Given data
- Initial volume (V₁): 16.44 L
- Initial temperature (T₁): 22.4°C + 273.15 = 295.6 K
- Initial pressure (P₁): 772.2 torr
- Final volume (V₂): 39.03 L
- Final temperature (T₂): 184.4°C + 273.15 = 457.6 K
We can find the final pressure applying the combined gas law.