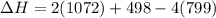Answer:
The correct answer is
B.
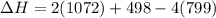
Step-by-step explanation:
Enthalpy of reaction :
It is the amount of energy released/absorbed when one mole of the substance is formed from the reactant at a constant pressure.
The enthalpy of a reaction can be calculated using :
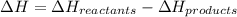
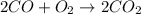
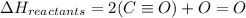
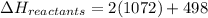
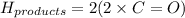
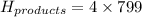
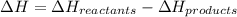
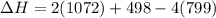
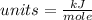
Please note that :
The carbon monoxide , CO should be taken as C triple bond O. Not C=O .
So , the bond energy =1072 is used