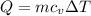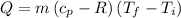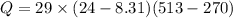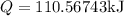During the process approximately 110 KJ heat will be absorbed by the gas.
Answer: Option D (110KJ)
Step-by-step explanation:
According to the question, the given values are,
The initial temperature,
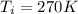
Let us assume that the final temperature will be
 , the initial pressure will be
, the initial pressure will be
 and the final pressure will be,
and the final pressure will be,
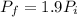
As we know that the Ideal Gas Equation at the constant volume is,
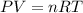
Then, for initial conditions,

And, for the final conditions,
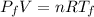
Now, on dividing both the equations, we get;
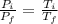
By putting all the values, we get,
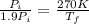
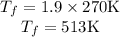
Hence, the heat absorbed at a constant volume V will be,