Answer:
Mass of Ni = 4.14 grams
Step-by-step explanation:
Molar mass : It is the amount of substance present in 1 mole of the molecules.
Moles : It is the quantity that contains as many atoms as present in 12 gram of C-12. This is equal to Avogadro Number .
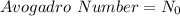
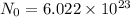
Molar mass of Ni = 58.69 g/mole
Moles can be calculated by two formula:
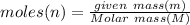
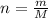 ........(1)
........(1)
And ,
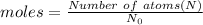
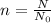 .........(2)
.........(2)
Comparing equation 1 and 2
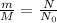
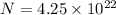
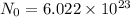
M = 58.69 g/mole
Put these value in the formula and solve for m
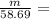
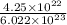
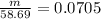
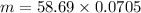
m = 4.14 gram
Mass of Ni = 4.14 grams
Second Approach :
Find the moles first using :
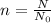
Then put value of n in the formula(1) and solve for m
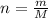 ........(1)
........(1)