Answer:
A) 3.59 cm
Step-by-step explanation:
Given that :-
The density of the gold ingot =
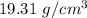
Given that:- Mass = 5.50 lbs
Also, considering the conversion of lbs to g as shown below:-
1 lb = 453.592 g
Thus,
Mass =
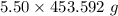 = 2494.756 g
= 2494.756 g
The volume = Length*Breadth*Height
Given that:- Length = 12.0 cm , Breadth = 3.00 cm
Considering the expression for density as:-
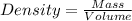
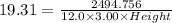
Solving for height, we get that:-
Height=3.59 cm