Answer:
We need to add
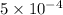 mg
mg
Step-by-step explanation:
Remember first:
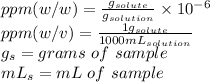
To calculate this mass, we need to calculate the concentration as follows:

Here we have the concentration of the sample in terms of x, being x the mg needed to have a solution of 1ppm (w/v). Therefore, we solve the system as follows:
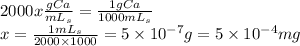
Finally we need
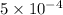 mg of sample to have a Ca concentration of 1ppm (w/v) in the final solution.
mg of sample to have a Ca concentration of 1ppm (w/v) in the final solution.