Answer: The average kinetic energy of the gas molecules will be proportional to the given temperature.
Step-by-step explanation:
Average kinetic energy is defined as the average of the kinetic energies of all the particles present in a system. It is determined by the equation:
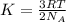
where,
K = Average kinetic energy
R = Gas constant
T = Temperature of the system
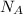 = Avogadro's number
= Avogadro's number
From the above relation, it is visible that kinetic energy is directly related to the temperature of the system. So, if average kinetic energy of the system increases, it means that temperature also increases and vice-versa.
Hence, the average kinetic energy of the gas molecules will be proportional to the given temperature.