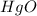Answer:
Moles of mercury = 0.00484 mol.
Moles of oxygen = 0.00481 mol.
The empirical formula is =
Step-by-step explanation:
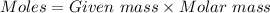
Mass of mercury = 0.971 g
Molar mass of mercury = 200.59 g/mol
Moles of mercury =
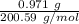 = 0.00484 mol.
= 0.00484 mol.
Since, the oxide contains only mercury and oxygen. SO,
Mass of O in the sample = Total mass - Mass of Hg
Mass of the sample = 1.048 g
Mass of O in sample = 1.048 g - 0.971 g = 0.077 g
Molar mass of oxygen = 15.999 g/mol
Moles of oxygen =
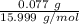 = 0.00481 mol.
= 0.00481 mol.
Taking the simplest ratio for Hg and O as:
0.00484 : 0.00481
= 1 : 1
The empirical formula is =