Answer:
Number of moles of CO2 = 88.01 mole
Step-by-step explanation:
Mole = It is the quantity that contains as many particles as present in 12 gram of C-12
There are two formula used to calculate number of moles :
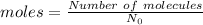
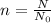
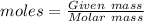
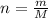
In this case use the formula in which number of molecules are given
The second formula is used in those case in which mass of the molecule is given
Given, Number of molecules (N)
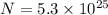
Avogadro number =
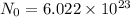
Number of moles can be calculated using :
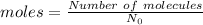
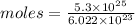
on dividing ,
Moles = 88.01 moles