Answer:
Mg(s) + 2HCl (aq) ⇒
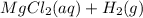
Step-by-step explanation:
Magnesium reacts with hydrochloric acid to produce magnesium chloride and hydrogen. This is a displacement reaction as magnesium displaces hydrogen from its compound HCl. This is because magnesium is higher than hydrogen in the activity series. Always try to balance the number of atoms on both sides of the equation after writing your equation.