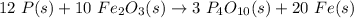Answer:
Step-by-step explanation:
Before writing this equation in a molecular equation form, let's identify each species:
- phosphorus is a non-metal represented by 'P', it is solid at standard conditions;
- iron(III) oxide is an ionic compound consisting of iron(III) cations with a 3+ charge, as well as of oxide anions with a 2- charge, this means we may represent it as
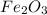 , which is also a solid material at standard conditions;
, which is also a solid material at standard conditions; - tetraphosphorus decoxide consists of 4 phosphorus atoms and 10 oxygen atoms, as stated by the prefixes, this is also a solid;
- iron metal is simply 'Fe'.
Putting all of this into a single equation and balancing it, we obtain: