Answer : When we increase the temperature of an exothermic reaction the equilibrium will shift to the left direction i.e, towards the reactant.
Explanation :
Le-Chatelier's principle : This principle states that if any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
As the given reaction is an exothermic reaction in which the heat is released during a chemical reaction. That means the temperature is decreased on the reactant side.
For an exothermic reaction, heat is released during a chemical reaction and is written on the product side.
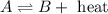
If the temperature is increases in the equilibrium then the equilibrium will shift in the direction where, temperature is getting decreased. Thus, the reaction will shift to the left direction i.e, towards the reactant.
Hence, when we increase the temperature of an exothermic reaction the equilibrium will shift to the left direction i.e, towards the reactant.