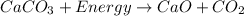Answer : The balanced decomposition reaction of
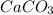 is:
is:
Explanation :
Endothermic reaction : It is defined as the chemical reaction in which the energy is absorbed from the surrounding.
In the endothermic reaction, the energy of reactant are less than the energy of product.
Exothermic reaction : It is defined as the chemical reaction in which the energy is released into the surrounding.
In the exothermic reaction, the energy of reactant are more than the energy of product.
Decomposition reaction : It is defined as the reaction in which the the larger molecule decomposes to give two or more smaller molecules.
It is represented as,
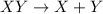
The balanced decomposition reaction of
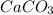 is:
is:
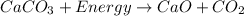
The decomposition reaction is an endothermic reaction in which energy is required during the reaction.
Hence, the balanced decomposition reaction of
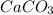 is:
is: