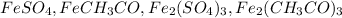Answer:
Step-by-step explanation:
Empirical formula of ionic compound formed by two ions
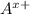 and
and
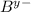 is
is
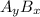 (for
(for
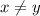 ) of AB (for x = y)
) of AB (for x = y)
The above empirical formula is in accordance with charge neutrality principle
Here each cation (
 and
and
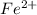 ) can form two ionic compounds by combining with two given anions (
) can form two ionic compounds by combining with two given anions (
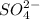 and
and
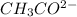 ).
).
So the four ionic compounds are: