Answer:
Change the temperature and pressure
Step-by-step explanation:
In case we are analyzing the solubility of a salt in some solvent, raising the temperature would increase the solubility of a salt generally. In contrast, at lower temperatures the solubility of ionic salts would decrease.
Now, another variation of this scenario might be solubility of gases. According to Henry's Law,
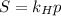 , meaning that the solubility of a gas is directly proportional to its partial pressure. Therefore, the greater the pressure, the greater the solubility of a gas.
, meaning that the solubility of a gas is directly proportional to its partial pressure. Therefore, the greater the pressure, the greater the solubility of a gas.