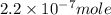Answer : The number of moles of hydronium ion is,
Explanation :
First we have tom calculate the concentration of hydrogen ion or hydronium ion.
pH : It is defined as the negative logarithm of hydrogen ion or hydronium ion concentration.
![pH=-\log [H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/8bj6kffduz54mxdb2y3cnu82gbhkta4kix.png)
Given:
pH = 7.35
![7.35=-\log [H_3O^+]](https://img.qammunity.org/2021/formulas/chemistry/college/8mqha6reqs9b0lix07qvbc7yyizosm9l28.png)
![[H_3O^+]=4.47* 10^(-8)mol/L](https://img.qammunity.org/2021/formulas/chemistry/college/jvpuns9rs10etlwjcwhy8uonc2vnjypdz3.png)
Now we have to calculate the moles of hydronium ion.
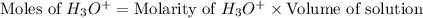
Now put all the given values in this expression, we get:
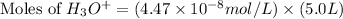
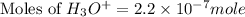
Therefore, the number of moles of hydronium ion is,