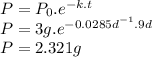Answer:
2.321 g
Explanation:
The half-life (t1/2) of phosphorus-32 is approximately 24.3 days. We can calculate the rate constant (k) using the following expression.
k = ln2 / t1/2 = ln2 / 24.3 d = 0.0285 d⁻¹
We have an initial amount of ³²P of 3 grams (P₀=3g). After a time t = 9 days, the mass remaining (P) can be calculated using the following expression.