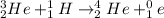Answer:
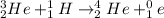
Step-by-step explanation:
In order to identify the missing species, we need to apply the law of mass and charge conservation. First of all, the total mass should be balanced. Assume that the unknown species is X with mass M and charge Z.
The total sum of masses of the reactants should be equal to the sum of masses of the products, meaning:
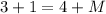
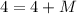
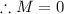
Similarly, apply the law for charges:

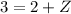
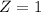
Therefore, the unknown particle has a mass of 0 and charge of 1. This is known as a positron: