Answer:
The heat exchange in the given process = -806.79 J
Step-by-step explanation:
0.235 moles of a diatomic gas expands doing 205 J of work while the temperature drops 88 K.
Number of moles , n = 0.235
Temperature difference = 88 K
According to conservation of energy,
ΔQ = ΔU + ΔW
We are given the amount of work done and we have to find the heat exchange in the process.
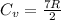 =
=
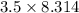 = 29.1
= 29.1
ΔU =
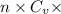 ΔT
ΔT
ΔU =
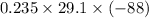 = -601.79 J
= -601.79 J
Work done by the gas = -205 J
ΔQ = ΔU + ΔW
Substituting the values,
ΔQ = -601.79 + -205 = -806.79 J
The heat exchange in the given process = -806.79 J