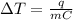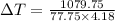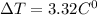Answer:
If 1,079.75 Joules of heat are added to 77.75 grams of water, by 3.32 degrees Celsius the temperature of water will increase
Step-by-step explanation:
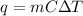
Here , q = heat added / removed from the substance
m = mass of the substance taken
 = Change in temperature
= Change in temperature
C = specific heat capacity of the substance
In liquid state the value of C for water is :
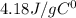
Given values :
q = 1079.75 J
m = 77.75 gram
Insert the value of C, m , q in the given equation
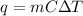
on transposing ,