Answer:
Percent Yield = 97.75 %
Step-by-step explanation:
1 MOLE = It is equal to the molar mass of the substance
1 mole of Cu = 63.54 g (Molar Mass of Cu = 63.54 g/mole)
1 mole of AgNO3 = 170 g (Molar Mass of AgNO3 = 170 g/mol)
Given Mass of AgNO3 = 1.41 g
Given Mass of Cu = 2.93 g
Second step : Find the limiting Reagent (which is in less amount)
Balanced Chemical equation :
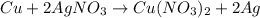
This means
1 mole of Cu will react with = 2 mole of AgNO3
63.54 g of Cu reacts with = 2 x 170 g of AgNO3
1 g of Cu reacts with = (2 x 170)/63.54 of AgNO3
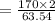
= 5.35 g of AgNO3
2.93 g should reacts with = 2.93 x 5.35 = 15.67 g of AgNO3
Available AgNO3 = 1.41 g
So , AgNO3 is less than required = limiting reagent
Now the reaction occur 1.41 g of AgNO3
Now, Limiting reagent will decide How much Silver(Ag) Metal will form
2 mole of AgNO3 will produce = 2 mole of Ag
1 mole of AgNO3 will produce = 1 mole of Ag
170 g AgNO3 will produce = 107.86 mole of Ag(Molar mass of Ag = 107.86)
1 g AgNO3 will produce =
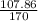
1.41 g of AgNO3 will produce =
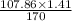
= 0.89 g
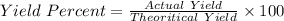
Actual yield = 0.87 g
Theoritical yield = 0.89 g
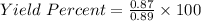
Percent Yield = 97.75 %