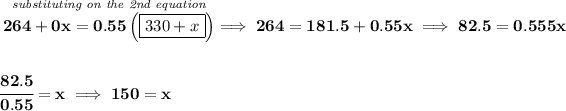y = amount needed at 55% alcohol.
x = amount of pure water, or 0% alcohol
so, you have on hand 330 mL of 80% alcohol, so how much alcohol only is there on that mixture? well, is 330 mL, so 80% of 330 is 330 * (80/100) = 330 * 0.8 = 264 mL.
likewise, let's convert the other perecentage values to deciaml, bearing in mind that pure water is, well just pure water, it has no alcohol, or we can say that it has 0% alcohol.