Answer:
Mg = 2,5 g; Al = 5,3 g
Step-by-step explanation:
1) Reactions
Mg + 2HCl ⟶ MgCl₂ + H₂
2Al + 6HCl ⟶ 2AlCl₃ + 3H₂
2) Mass of each metal
If there had been no reaction, the mass of the solution would have increased by 7,8 g.
The mass increased by only 7,0 g.
The missing 0,8 g must represent the mass of the hydrogen generated by the reaction.
We have two relations:
Mass of Mg + mass of Al = 7,8 g
H₂ from Mg + H₂ from Al = 0,8 g
i) Calculate the moles of H₂
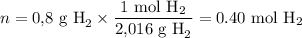
(ii) Solve the relationship
Let x = mass of Mg. Then
7,8 - x = mass of Al
Moles of Mg = x/24.30
Moles of Al = (7,8 - x)/26.98
Moles of H₂ from Mg = (1/1) × moles of Mg = 1 × (x/24,30) = 0,0412x
Moles of H₂ from Al = (3/2) × Moles of Al = 1.5(7,8 - x)/26,98 = (11,7 -1,5x)/26,98
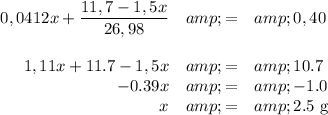
Mass of Mg = 2,5 g
Mass of Al = 7,8 g - 2,5 g = 5,3 g
The masses of the metals are Mg = 2,5 g; Al = 5,3 g