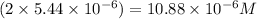Answer: Hence, the equilibrium concentration of the
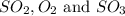 are 0.521, 0.632 and
are 0.521, 0.632 and
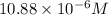 respectively.
respectively.
Step-by-step explanation:
The balanced chemical reaction is:
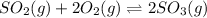
At t = 0 0.522 0.633 0
At
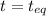 0.522-x 0.633-2x 2x
0.522-x 0.633-2x 2x
The expression for
 for the given reaction follows:
for the given reaction follows:
![K_c=([SO_2]^2)/([SO_2]* [O_2])](https://img.qammunity.org/2021/formulas/chemistry/high-school/l4du0pajxsielm83zlw99mopc7y4q29d14.png)
We are given:
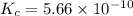
Putting values in above equation, we get:
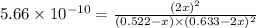
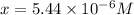
Thus equilibrium concentration of
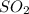 is = (0.522-x )=
is = (0.522-x )=
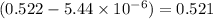
equilibrium concentration of
 is = (0.633-2x )=
is = (0.633-2x )=
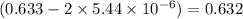
equilibrium concentration of
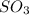 is = (2x )=
is = (2x )=