Answer:Increases
Step-by-step explanation:
As the temperature increases average kinetic energy of particle is increases.
As the temperature increases molecules started to vibrate at higher frequency which causes to rise in temperature. Mathematically, average kinetic energy is given by
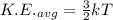
where k=boltzmann constant
T=temperature