Answer:
c 3 mol of Ba(OH)₂ and 1 mol of Ba₃N₂ should be interchanged
Step-by-step explanation:
Ba₃N₂ + 6H₂O → 3Ba(OH)₂ + 2NH₃
n/mol: 9.2
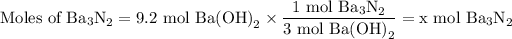
They want to find moles of Ba₃N₂, so that should be in the numerator of the conversion factor,
Moles of Ba(OH)₂ should be in the denominator to cancel the units in 9.2 mol Ba(OH)₂,