Answer:
10 g of water at
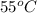
Step-by-step explanation:
According to the kinetic molecular theory, the kinetic energy of a particle depends only on temperature. This is described by the following equation:
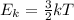 . Notice that the kinetic energy term is directly proportional to the absolute temperature.
. Notice that the kinetic energy term is directly proportional to the absolute temperature.
The average kinetic energy is independent of mass, meaning when we examine each of these samples, we are only looking for a sample that has the greatest temperature. Out of all the samples, the sample at
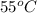 is at the highest temperature which implies it has the greatest average kinetic energy.
is at the highest temperature which implies it has the greatest average kinetic energy.