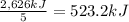Answer:
The net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide is 523.2 kJ.
Step-by-step explanation:
Step 1:
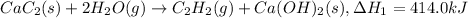 ...[1]
...[1]
Step 2 :
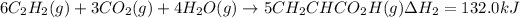 ..[2]
..[2]
Adding 6 × [1] and [2]:
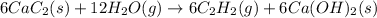
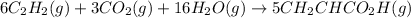
we get :
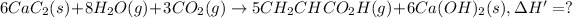

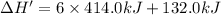
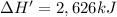
Energy released on formation of 5 moles of acrylic acid = 2,626 kJ
Energy released on formation of 1 mole of acrylic acid: